Stryker STAR™ Total Ankle
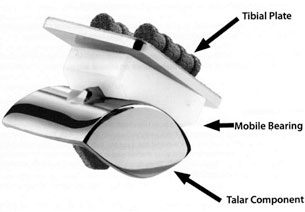
The U.S. Food and Drug Administration (FDA) has approved SBi’s Scandinavian Total Ankle Replacement (STAR™ Ankle) system to treat U.S. patients. It is a safe, proven alternative to failed conservative treatment. It is the only three piece mobile bearing non constrained, uncemented total ankle replacement (to receive pre-market approval to replace a painful arthritic ankle joint due to post traumatic arthritis or rheumatoid arthritis).
The instructions for use and patient labeling issued by the FDA indicates the STAR™ Ankle patients had superior effectiveness compared to ankle fusion and had comparable safety results to ankle fusion in the clinical trial.
The STAR™ Ankle has more than 19 years of clinical experience and the current design has been implanted in over 15,200 patients worldwide. Additionally, there have been 35 peer-reviewed clinical outcome papers published on the STAR™ Ankle. SBi believes that this number of papers is more than any other mobile-bearing total ankle arthroplasty device.
- Visit Website: Stryker STARTM Total Ankle
- Download Brochure: Stryker STAR™ Total Ankle Brochure
